Molecular alterations The most frequently mutated and/or amplified genes in the tumour cells are TP53 (41% of tumours), PIK3CA (30%), MYC (20%), PTEN (16%), CCND1 (16%), ERBB2 (13%), FGFR1 (11%) and GATA3 (10%), as reported in a series of early breast cancers. These genes encode cell-cycle modulators that are either repressed (for example, p53) or activated (for example, cyclin D1), sustaining proliferation and/or inhibiting apoptosis, inhibiting oncogenic pathways that are activated (MYC, HER2 and FGFR1) or inhibiting elements that are no longer repressed (PTEN).
The majority of the mutations affecting 100 putative breast cancer drivers are extremely rare, therefore, most breast cancers are caused by multiple, low-penetrant mutations that act cumulatively. Luminal A tumours have a high prevalence of PIK3CA mutations (49%), whereas a high prevalence of TP53 mutations is a hallmark of basal-like tumours (84%).
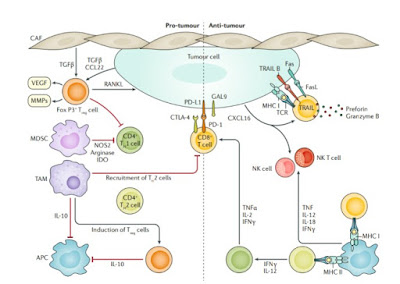
For TNBC, different molecular drivers under- line its subtypes. At the metastatic stage, specific predictive alterations, such as PIK3CA mutations, can be easily detected non-invasively in the plasma in circulat- ing tumour DNA rather than on tumour biopsy; never- theless, depending on the technology used, the level of sensitivity may vary.
Epigenetic alterations are involved in breast carcinogenesis and progression. In breast cancer, genes can be either globally hypomethylated (leading to gene activation, upregulation of oncogenes and chromosomal instability) or, less frequently, focally (locus-specific) hypermethylated (leading to gene repression and genetic instability due to the silencing of DNA repair genes).
Other epigenetic mechanisms involve histone tail modifications by DNA methylation, inducing chromatin structure changes to silence gene expression and nucleosomal remodelling. These changes are reversible, enzyme-mediated and potentially targetable. For example, in luminal-like breast cancer cell lines, inhibition of histone deacetylase with specific inhibitors such as vorinostat or chidamide can reverse resistance to endocrine therapy via inhibition of the resistance pathway driven by epidermal growth factor receptor signalling.
Recently, a phase III trial in metastatic luminal breast cancer showed the superiority of a treatment combining chidamide with endocrine therapy (namely, the aromatase inhibitor exemestane) to exemestane alone.