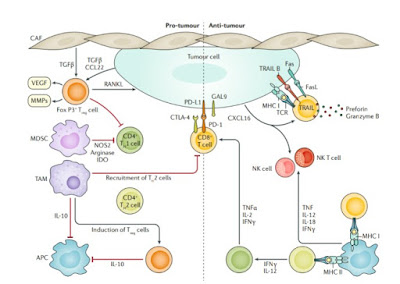
Immune crosstalk in breast cancer. The immune reaction to breast cancer is initiated by the neoantigens expressed by tumour cells, encoded by altered genes and presented by antigen-presenting cells (APCs) on major histocompatibility complex class I (MHC I) or MHC II molecules. Neoantigen presentation results in activation of CD8+ (cytotoxic) and CD4+ (helper) T cells. CD8+ T cells are the main effector cell of the anti-tumour immune response; their activation (principally through the T cell receptor (TCR)) results in release of the cytolytic molecules perforin and granzyme B, which directly induce tumour cell lysis.
The anti-tumour action of CD8+ T cells is amplified by cytokines secreted from CD4+ T cells, namely IFNγ, IL-2 and tumour necrosis factor (TNF). Activated CD8+ T cells also upregulate expression of Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL; also known as TNFSF10) on their membrane, which induce apoptotic pathways to kill tumour cells.
Cancer cells elicit an innate immune response, comprising natural killer (NK) and NK T cells that are capable of direct tumour cell killing. Malignant cells can suppress the immune response by expressing immune checkpoint regulators (for example, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 ligand 1 (PD-L1)), which are upregulated by effector T cells as a consequence of chronic exposure to tumour antigens (T cell exhaustion).
The reduced anti-tumour immune response by upregulated immune checkpoint molecules establishes a pro-tumour microenvironment, which is further enriched by recruitment of immunosuppressive cells, T regulatory (Treg) cells and myeloid-derived stromal cells (MDSCs). Treg cells, which inhibit activation of CD4+ and CD8+ T cells, are induced by tumour-associated macrophages (TAMs) and by tumour-secreted and cancer-associated fibroblast (CAF)-secreted factors, such as transforming growth factor-β (TGFβ).
In addition, TAMs and Treg cells inhibit APCs via IL-10 secretion, inducing a tolerogenic state of APCs. MDSCs are recruited to the tumour bed by tumour-secreted factors, inhibit trafficking of T cells to the tumour bed and inhibit effector T cell activation by upregulating 2,3-indoleamine- dioxygenase (IDO) and arginase expression, enzymes involved in the T cell nutrient depletion.
The secretome of the pro-tumour microenvironment, containing factors that stimulate angiogenesis and invasion (such as vascular-endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs)) also contribute to tumour immune escape and propagation. CCL22, CC-chemokine ligand 22; CXCL16, CXC-chemokine ligand 16; NOS, nitric oxide synthase; PD-1, programmed cell death 1; RANKL, receptor activator of nuclear factor-κB (RANK) ligand; TH1 cell, type 1 T helper cell. Adapted from ref. 75, CC-BY-4.0 https://creativecommons.org/licenses/by/4.0/